Abstract
Background
Imatinib mesylate (IM) 400 mg daily still represents the gold standard for chronic phase chronic myelogenous leukemia (CP-CML). Currently, the initiation dose of IM is adapted to the body weight only in childhood. There are some concerns regarding the adaptation of IM prescription in overweighted patients that may respond poorly to this tyrosine kinase inhibitor (TKI). However, this has not been extensively studied in the first-line setting. In addition, it is known that IM is able to promote weight gain and further, obesity has been identified as a possible risk factor in several solid cancers and leukemias.
Aims
In a retrospective observational monocentric study, we evaluated the impact of the weight and the body mass index (BMI) measured at diagnosis on CP CML patients outcomes, on IM first-line, in terms of survival, responses and kinetics.
Methods
We retrospectively enrolled in this study, newly diagnosed CP CML patients, treated with IM in first-line between 2000 and 2017. Overall survival (OS), progression-free survival (PFS), failure-free survival (FFS, defined as progression to advanced phases death, loss of CHR, CCyR, or MMR, discontinuation of IM for toxicity, primary cytogenetic resistance) were analysed since IM initiation in intention-to-treat. Responses (hematologic, cytogenetic, molecular) were analysed according to standard methods. Molecular results were standardised according to the ELN/Eutos programs since 2003, and were expressed as BCR-ABL (IS) in %. Clinical data were extracted from medical files, weight and height measured for all patients at diagnosis, and later nearly all patients being weighted at each time point. The BMI was categorized into 4 stages according to the OMS classification: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI ≤ 24.9), overweight (25 ≤ BMI ≤ 29.9), and obesity (BMI ≥ 30). Serum Albumin (g/l) was regularly assessed, and the nutritional risk index (NRI) was also calculated.
Results
We enrolled 107 patients in this study, 62 (58%) males, with a median age at diagnosis of 55.36 (17-83) years. Sokal risk and Hasford scores were low for 32% and 39%, Intermediate for 48% and 52%, and high for 20% and 9% respectively, (2 patients unknown). Eutos score was low for 95% of patients. The median BMI at diagnosis was 24.61 (17-44.3) Kg/m2. The median weight at diagnosis was 69 (48-128) Kg. We analysed the patients in 2 groups: A: low + normal BMI (<24.9) and B: overweighted + obese (>25). The median follow-up was 97.3 (3-17) months. Age at diagnosis, Sex ratio, duration on IM, Sokal, Euro, Eutos risk scores, presence of ACA at diagnosis, menopauses women, IM duration were not different between groups A & B. There was significantly more diabetic patients in group B (3 vs 11, p=0.018). The rates of CCyR at 6 months, MMR at 12 months were not different between the 2 groups (38% in group A vs 48.9% in group B, p=0.388; and 48.94% in group A vs 66.67% in group B, p=0.133). The rate of MR4 (56 [44.09-71.91]% group A vs 67.7 [52.60-80.74]% group B, p=0.817), MR4.5 (48 [33.95-62.05]% group A vs 48.9 [34.05-63.73]% group B, p=0.97) at 5 years were not significantly different.
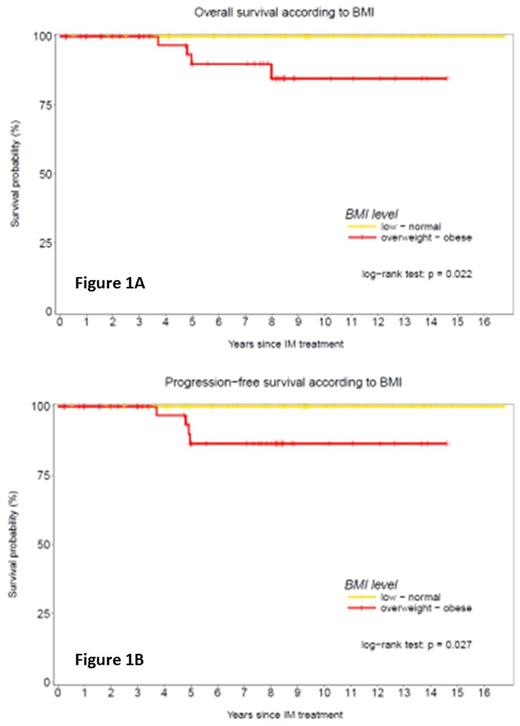
Five patients died, all in group B, from a lymphoid blast crisis in 1 patient, secondary tumours in 3 (lung, colon, prostate cancer + cholangiocarcinoma) and terminal renal insufficiency in 1. The OS rate at last follow-up was significantly better in low/normal BMI 100% months (A) versus 84.7 [71.5-100] % months (B, p=0.022) (Fig 1A), as well as the PFS 100% months versus 86.5 [75.-99.7] % months (p=0.027) (Fig 1B).
The FFS rate was not different (p=0.794). Interestingly, 25 (23.4%) patients fulfilling the STIM criteria for Treatment-Free Remission, were 17 in arm A and 8 in arm B. Of note, the BMI regularly increases over time with an increase of 0.045 [0.006-0.084] Kg/m2 per month, and patients may become overweighted over time (42% at diagnosis, 45.8% at latest follow-up).
Conclusion
This retrospective analysis on an homogeneous population demonstrates that being overweighted at diagnosis of CP CML, and undergoing IM treatment first-line, selectively impacts on survival. However, the rates of responses do not seem to be altered, and the majority of such patients died from CML unrelated causes, mostly from other cancers. The mechanisms responsible for that are probably complex, multifactorial, and confounding factors may interfere, and finally remain to be deciphered.
Labussiere-Wallet: Novartis: Speakers Bureau. Nicolini: Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte Biosciences: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; ARIAD: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal